Medicaid Rule Undermines Insulin Access
Medicaid’s efforts to save a buck could cost diabetes patients in a big way.
New “Fast Facts” Recaps Biosimilar Policy Developments
Given the Trump administration’s rapid succession of proposals, policy plans and regulatory developments, 2018 may well be the year of biosimilars policy.
Event Aims to Encourage, Empower Gout Patients
Painful, debilitating and highly stigmatized, gout affects 8-9 million Americans. The disease occurs when uric acid builds up in the joints, triggering agonizing attacks and disrupting people’s day-to-day lives. But the Alliance for Gout Awareness has a message for frustrated patients: Don’t give up.
New Research Sheds Light on Women & Migraine
When it comes to migraine and headache disorders, women have clear-cut opinions – and distinctly female experiences.
FDA Hearing Explores Competition & Innovation in Biosimilars

When it comes to how to increase the development and utilization of biosimilars, the Food and Drug Administration is all ears. On Tuesday, the agency welcomed numerous physicians and experts to testify on competition and innovation in the biosimilars marketplace. And at least one physician identified a critical component in uptake: physician confidence.
Who Has a Seat at ICER’s Table?
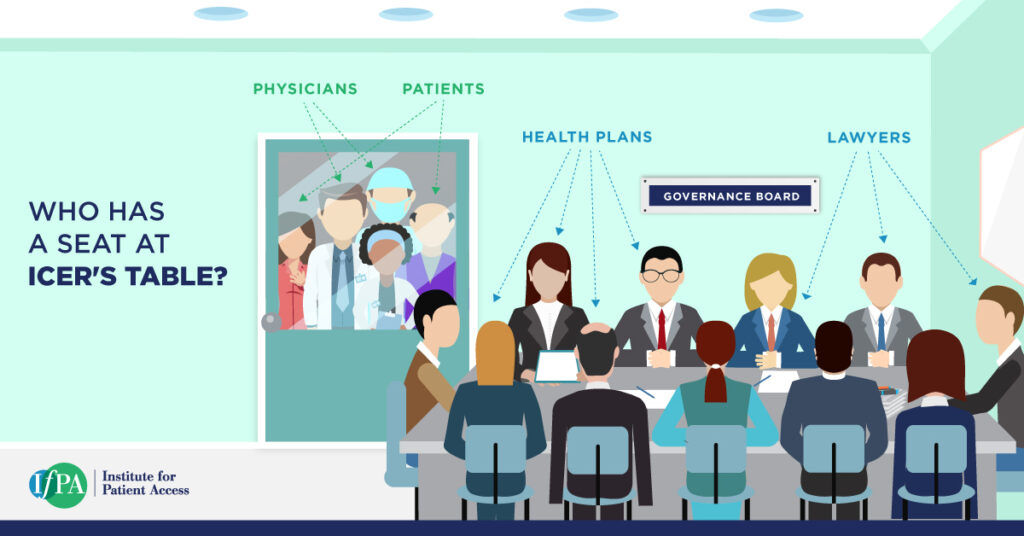
ICER claims to define the value of medicine for patients. But who’s defining ICER’s values?
Illinois Joins Maine in Limiting Non-Medical Switching

What do Maine and Illinois have in common? Legislators in both states are heeding patients’ concerns about non-medical switching this year.
Summer 2018: A Health Policy Recap in 10 Quotes

Patient access developments didn’t stop for summer. As we head into fall, here are 10 quotes from the news items – and new materials – that kept the health policy conversation lively during summer 2018.
Patient Access Withers Under ICER’s Influence

CVS Health recently announced that employers who use its pharmacy benefit management services can cut certain medications from their plan’s formulary of approved drugs. Which drugs? Those that exceed a cost-effectiveness threshold established by ICER, the Institute for Clinical and Economic Review.
How Latin America Can Avoid a Health Care Misstep

When public health emergencies threaten developing nations, governments may look for cheap, fast ways to secure necessary medicine. One option is compulsory licensing, the topic of a new “Fast Facts” from the Global Alliance for Patient Access.

