What Cancer Drugs Might Mean for Alzheimer’s and Parkinson’s Patients
Patients with Alzhe.imer’s or Parkinson’s disease may have a new source of hope: cancer drugs
FDA Hearings Underscore Need for Clear Policy on Biosimilars
July 12 and 13 hearings of the FDA’s Arthritis Advisory Committee cleared the way for two new biosimilars, adalimumab and etanercept. The committee unanimously supported manufacturers’ applications for the drugs – but that wasn’t the only point of consensus. Participants and even committee members expressed concerns about unfinished regulatory policy and the need to prioritize patient safety.
Senators Urge Action on National Pain Strategy
A bipartisan group of U.S. senators has a question for HHS Secretary Sylvia Burwell: Any progress on the National Pain Strategy? In a letter to Secretary Burwell, 12 senators reiterate the widespread suffering and financial toll of chronic pain. “It is time,” they explain, “to transform the [National Pain Strategy] from a plan on paper to a reality …to improve [Americans’] health and quality of life.”
AfPA Releases Video on Barriers to Asthma Care
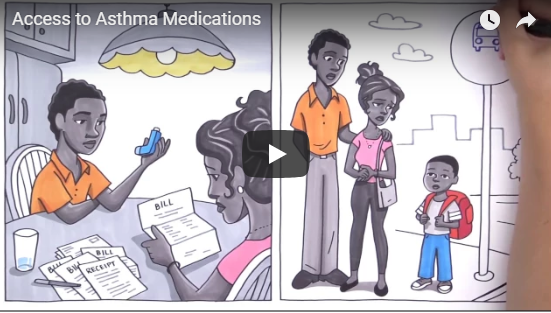
Breathing complications aren’t the only challenge faced by the 26 million Americans with asthma, explains a new video from the Alliance for Patient Access.
CMS Models Shake Up Cancer Care
The Centers for Medicare and Medicaid Services’ Oncology Care Model is off and running. As announced during The White House’s cancer summit at Howard University, more than 200 physician practices and 3,200 oncologists have enrolled – effective July 1.
Global Poster Presentations Convey Need for Distinct Biosimilar Names
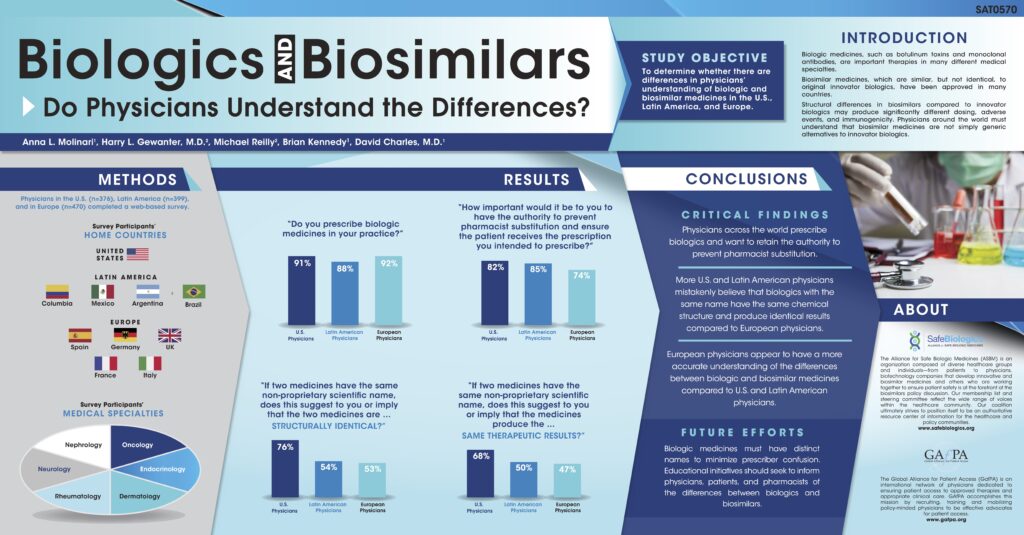
Patient advocates have teamed up to take an important message to European physicians and policymakers: distinct names for biosimilar medications are essential.
Delaware Joins States Improving Patient Access to Hepatitis C Cures
Starting July 1, patients battling hepatitis C in Delaware will have newfound access to direct-acting antiviral cures – a victory now shared with patients in New York, Pennsylvania, Florida and Washington.
State Efforts to Curb Opioid Abuse Could Increase Patients’ Pain
The opioid abuse epidemic continues to generate state laws aimed at reducing opioid misuse and overdose.
A Reality Check on Health Care Value Models
High drug prices have led to a proliferation of value models that investigate how the United States can control ballooning health care costs. But just how realistic are these models – and their implementation?
Labeling Debate Hones in on Biosimilarity Statement
Stakeholders are divided over a provision of the Food and Drug Administration’s draft regulatory guidance on biosimilar labeling, letters to the FDA reveal.

