New “Fast Facts” Recaps Biosimilar Policy Developments
Given the Trump administration’s rapid succession of proposals, policy plans and regulatory developments, 2018 may well be the year of biosimilars policy.
Health Plan Coverage for Biosimilars Raises Questions

Do coverage decisions aim to offer patients more treatment options – or drive patients to non-identical drugs based on cost alone?
EU Group Calls for Greater Transparency in Labelling Biosimilars

EU regulators who want to encourage understanding and acceptance of biosimilar medicines might consider improving the drugs’ labels.
New AfPA Video Examines “The Interchangeability Promise”

First came biologics, then biosimilars. And soon, explains a newly released video from The Alliance for Patient Access, patients can expect interchangeable biosimilars.
Patient Safety Questions Linger after FDA Issues Interchangeability Guidance
New guidance from the Food and Drug Administration directs companies on how to demonstrate a biosimilar’s interchangeability with its reference product.
There’s no way to address America’s prescription drug abuse crisis without balanced pain management, explained the National Institutes of Health’s Linda Porter, PhD, at Tuesday’s Summit on Balanced Pain Management.
f policymakers get it right, biosimilars could offer more safe and effective treatment options for patients in the coming years – potentially at lower costs.
Physicians Align on Meaningful Biosimilar Suffixes

The Food and Drug Administration may not have reached consensus on the suffixes that distinguish biological and biosimilar medications, but physicians are of one mind: Meaningful suffixes matter.
Nordic Patient Groups Tackle Biosimilar Policy Issues

Biosimilar medicines are being prescribed more and more freely across Europe. But access to, and use of, these new medicines differs greatly depending on which European country you live in.
Global Poster Presentations Convey Need for Distinct Biosimilar Names
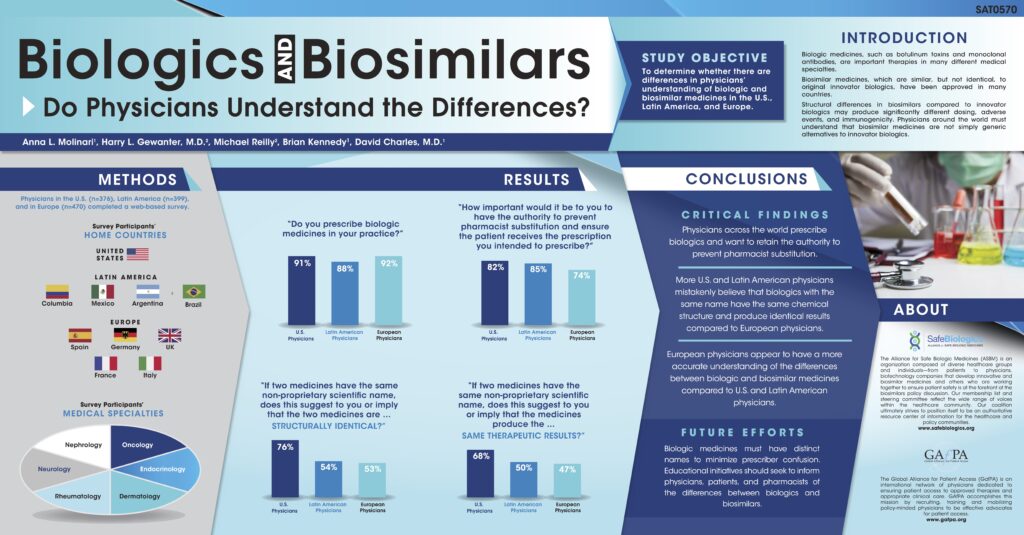
Patient advocates have teamed up to take an important message to European physicians and policymakers: distinct names for biosimilar medications are essential.
Senate Bill Could Eliminate Potential Access Barrier for Biosimilars
The Senate companion to last year’s 21st Century Cures Act could help biosimilars bypass a potential roadblock to patient access.

