Alliance for Patient Access Assumes Leadership of Rx Process Website
The Alliance for Patient Access (AfPA) today announced that it will now manage www.PrescriptionProcess.com, an online resource for patients to learn, share stories and advocate about access to approved medical therapies.
New Study Calls for Informed Prescribing to Further Patient Access
A new study from the Food and Drug Law Journal argues that fewer restrictions on communication between pharmaceutical companies and physicians would foster better-informed prescribing decisions.
BIOtechNOW Spotlights the Coalition for Clinical Trials Awareness
In a new BIOtechNOW blog post, Coalition for Clinical Trials Awareness (CCTA) steering committee members explain how stalled clinical trials stifle patients’ hope for breakthrough medicines.
Don’t Keep Physicians Guessing About Biosimilar Swaps

State legislatures across the country are considering bills to ensure that physicians know when a pharmacist swaps a prescribed biologic medicine for a new “biosimilar.”
Patients Need Access to Reap the Benefits of Abuse-deterrent Pain Meds

As most Americans now realize, abuse of prescription pain medication presents a formidable challenge.
Biosimilar Labeling Stirs Concerns

Will the labels used for biosimilars help – or hinder – the adoption of these new medications?
IfPA Policy Brief Identifies Barriers to Integrated Pain Care
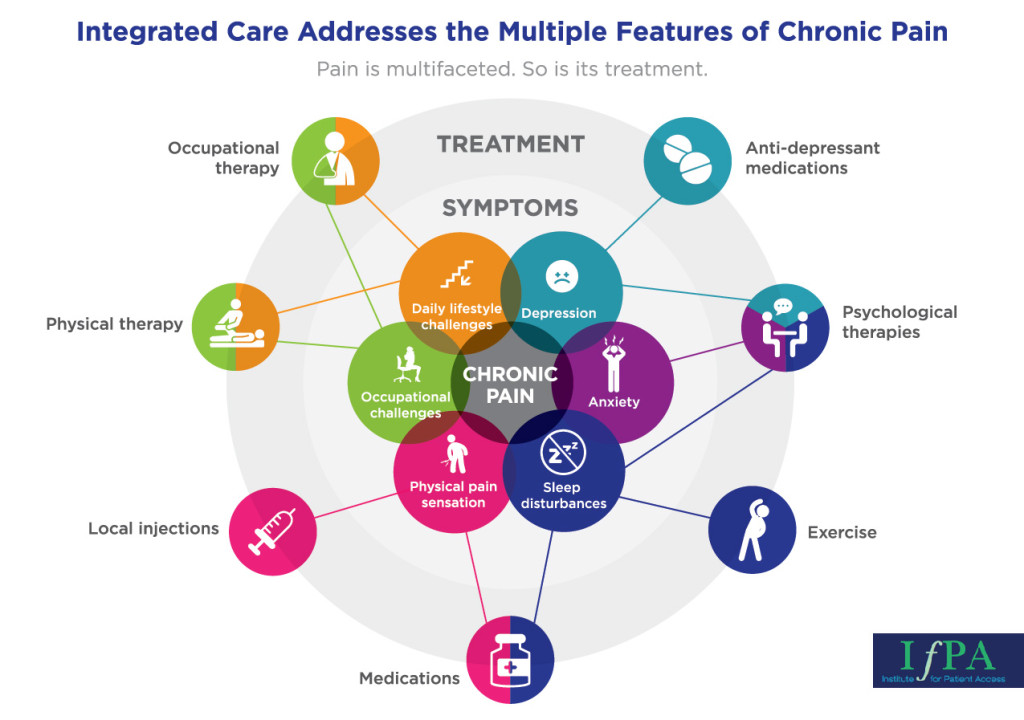
Chronic pain’s multifaceted symptoms require a multidisciplinary approach to care, says the Institute for Patient Access’ new policy brief. Yet several barriers may prevent chronic pain patients from finding the long-term relief they need.
Will New Medicare Payment Model Hurt Individualized Cancer Care?
A new oncology payment model rewards “value of care, not volume” says the Centers for Medicare and Medicaid Services.
First Biosimilar Approval Leaves Patient Safety Questions Unanswered
On Friday the Food and Drug Administration approved the United States’ first biosimilar medicine.
Co-infected Hepatitis C Patients Find New Options, New Obstacles
Patients co-infected with hepatitis C and HIV may have newfound hope. Clinical trial results presented at the 2015 Conference on Retroviruses and Opportunistic Infections show that a new combination pill worked for co-infected patients of certain genotypes 96 percent of the time.

