Who decides which medications are available to the 5 million Americans living with Alzheimer’s?
Increasingly, questions of access are informed by the Institute for Clinical and Economic Review, or ICER. The health economics organization uses clinical trials data and available pricing information to consider whether a drug is worth its cost.
Now, ICER is reviewing a new treatment for Alzheimer’s disease. ICER’s upcoming review will examine a medication, Aducanumab, that could be the first disease modifying treatment approved to treat Alzheimer’s disease.
Get Involved
ICER’s reports can influence health plans’ decisions about coverage. Input from advocacy organizations, health care providers and patients is critical.
Register for ICER’s Public Meeting
ICER will host a public meeting on July 15 to deliberate and vote on evidence presented in their report on Alzheimer’s disease.
Share Social Media Graphics
To download and share: Click an image above, then right click and choose “save image as”. Choose a destination on your computer, such as your desktop or a folder, and click save. Then, post to Facebook or Twitter just as you would any other image.
Tweet & Post to Raise Awareness
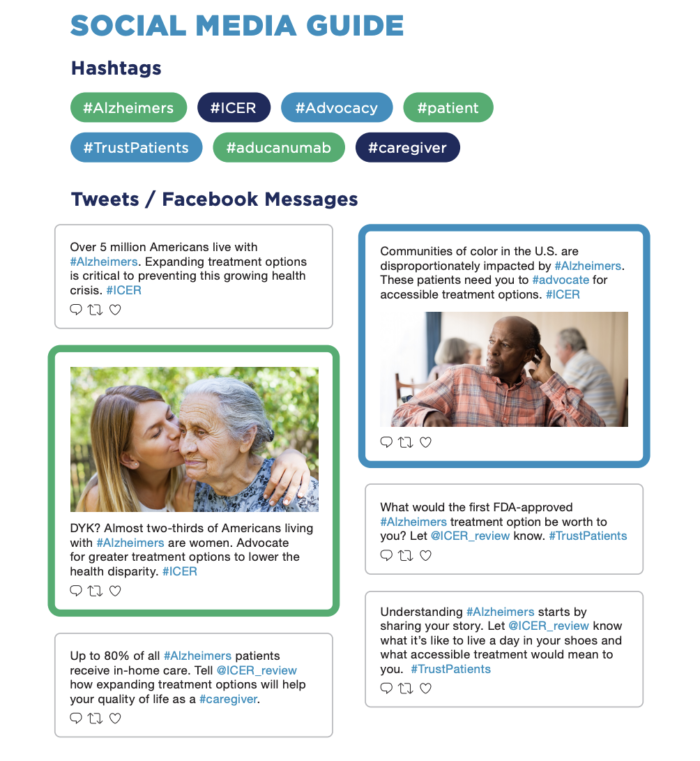
- Over 5 million Americans live with Alzheimers. Expanding treatment options is critical to preventing this growing health crisis. #ICER
- DYK? Almost two-thirds of Americans living with #Alzheimers are women. Advocate for greater treatment options to lower the health disparity. #ICER
- Up to 80% of all #Alzheimers patients receive in-home care. Tell @ICER_review how expanding treatment options will help your quality of life as a #caregiver.
- Communities of color in the U.S. are disproportionately impacted by #Alzheimers. These patients need you to #advocate for accessible treatment options. #ICER
- What would the first FDA-approved #Alzheimers treatment option be worth to you? Let @ICER_review know. #TrustPatients
- Understanding #Alzheimers starts by sharing your story. Let @ICER_review know what it’s like to live a day in your shoes and what accessible treatment would mean to you. #TrustPatients
Learn More

Who Will Control Access to New Alzheimer’s Treatments?
An Institute for Patient Access blog explains that a “poorly structured review could delay patient access to this – and future – treatments that represent the best hope yet against this still incurable disease.”
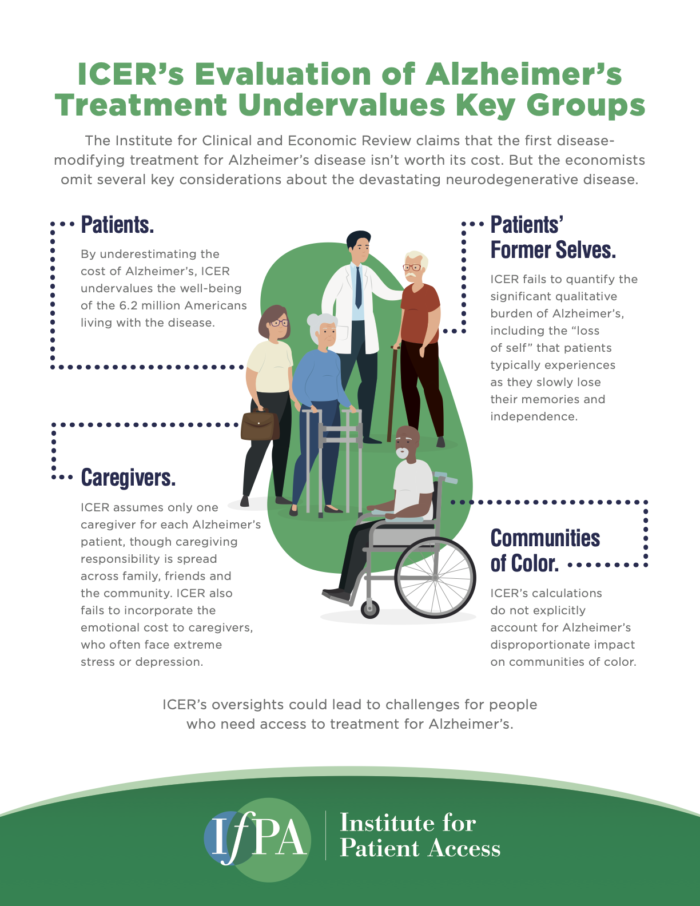
ICER’s Evaluation of Alzheimer’s Treatment Undervalues Key Groups
The Institute for Clinical and Economic Review claims that the first disease-modifying treatment for Alzheimer’s disease isn’t worth its cost. But the economists omit several key considerations about the devastating neurodegenerative disease.